What Is the Noble Gas Configuration for Barium
The energy needed to remove the most loosely held electron from a neutral atom is called 1st ionization. The attraction of an atom for an additional electron is called electron affinity.

Ba 2 Electron Configuration Barium Ion Youtube
Oxidation States Oxidation states are typically represented by integers which may be positive zero or negative.
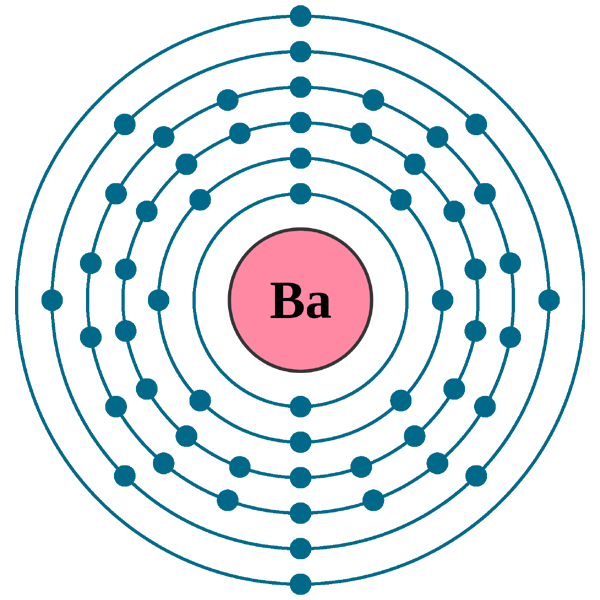
. Melting point The temperature at which the solidliquid phase change occurs. The electron configuration can be visualized as the core electrons equivalent to the noble gas of the preceding period and the valence electrons eg. Xe 6s2 for barium.
Noble gas configuration Noble gases Nonmetals Semimetal Shielding effect 1. What is the correct systematic name for Cs. Write all the possible sets of magnetic quantum numbers m_l for an electron in the n.
This strongly supports the concept that the electronic configuration of a Noble Gas group 18 element is remarkably stable and that any atom or ion with this structure will not be chemically reactive. 19 1s22s22p63s23p64s24d104p5 not valid take a look at 4d 20 1s22s22p63s23d5 not valid 3p comes after 3s 21 Ra 7s25f8 not valid radium isnt a noble gas 22 Xe not valid an element cant be its own electron configuration. At this point however the ordering of orbitals becomes more complex than it previously had been because there are now unfilled 4f orbitals as well as the 5d orbitals and the two sets have approximately the same energy.
It is in group 18 of the periodic table and is a noble gas. Sublimation The transition of a substance directly from the solid to the gas phase without passing through a liquid phase. Electron Configuration Practice Worksheet KEY.
Ionization energy is the energy required to remove an electron from an atom. Oxidation States Oxidation states are typically represented by integers which may be positive zero or negative. A noble gas electronic configuration.
The electron configuration can be visualized as the core electrons equivalent to the noble gas of the preceding period and the valence electrons eg. The electron configuration can be visualized as the core electrons equivalent to the noble gas of the preceding period and the valence electrons eg. What is the correct.
In the next element lanthanum atomic number 57 an. What is the electrical attraction between oppositely charged chemical species. This is a barium meal or barium enema.
Electron configuration The arrangements of electrons above the last closed shell noble gas. All barium compounds are toxic. Barium is a heavy element and scatters X-rays so as it passes through the body the stomach and intestines can be.
Argon is the third-most abundant gas in the Earths atmosphere at 0934 9340 ppmvIt is more than twice as abundant as water vapor which averages about 4000 ppmv but varies greatly 23 times as abundant as carbon dioxide 400 ppmv and more. In the space below write the unabbreviated electron configurations of the following elements. Boiling point The temperature at which the liquidgas phase change occurs.
18 Xe 6s2 barium These electron configurations have mistakes determine what is wrong. 5 But why is it easier to remove these valence electrons as you go down group. As a result Group 2 elements form ionic compounds in which the group 2 cation has a charge of 2.
Argon is a chemical element with the symbol Ar and atomic number 18. Noble gas configuration is the electron configuration of noble gases. When the configuration of the noble gas krypton has been achieved.
What is the sharing of electrons between 2 atoms. A suspension of barium sulfate is sometimes given to patients suffering from digestive disorders. What is the correct systematic name for Ba2.
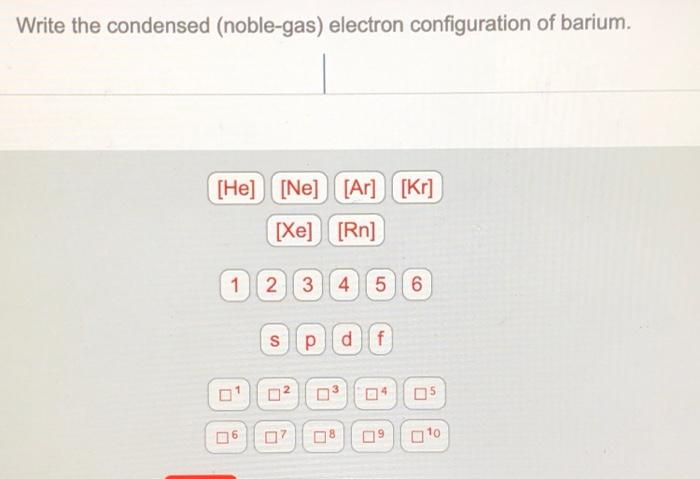
Xe 6s2 for barium. Write the noble gas electron configuration for barium. Xe 6s2 for barium.
The noble gases He Ne Ar Kr Xe Rn are less reactive than other elements because they already have a noble. Group 6A nonmetals form ----2 ions. Periodic Table With Electron Configuration Alkali Metal Alkaline Earth Transition Metal Basic Metal Metalloid Nonmetal Halogen Noble Gas Lanthanide H Hydrogen 1008 1 Li Lithium 6941 Beryllium 2 1 Mg Magnesium 24305 2 8 1 2 8 2 K Potassium 39098 Ca.
The basis of all chemical reactions is the tendency of chemical elements to acquire stability. Main-group atoms generally obey the octet rule while transition metals generally obey the 18-electron rule. However barium sulfate is insoluble and so can be safely swallowed.
Oxidation States Oxidation states are typically represented by integers which may be positive zero or.

Ba Barium Element Information Facts Properties Trends Uses And Comparison Periodic Table Of The Elements Schoolmykids
A Step By Step Description Of How To Write The Electron Configuration For Barium Ba Youtube

Barium Electron Configuration Ba With Orbital Diagram

Solved When Writing The Noble Gas Configuration For Barium Chegg Com
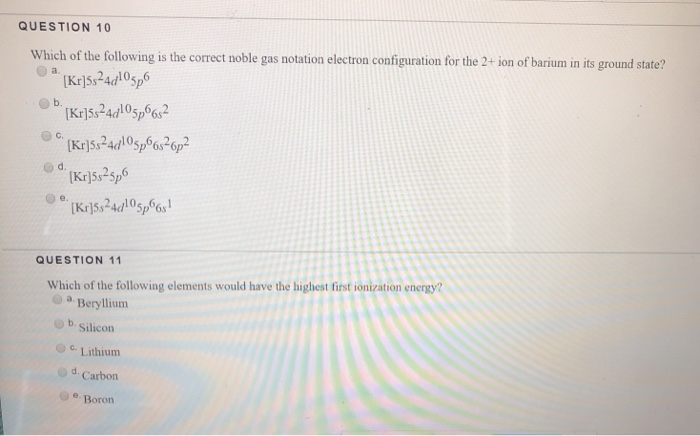
Solved Question 10 Which Of The Following Is The Correct Chegg Com

A Barium Atom Attains A Stable Electron Configuration When It Bonds With Lisbdnet Com

Answered Write The Condensed Noble Gas Bartleby

Barium Ba Electron Configuration And Orbital Diagram
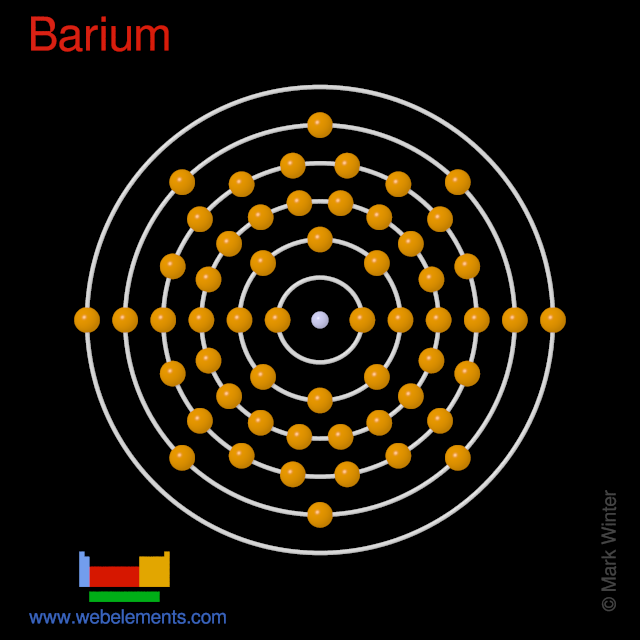
Webelements Periodic Table Barium Properties Of Free Atoms
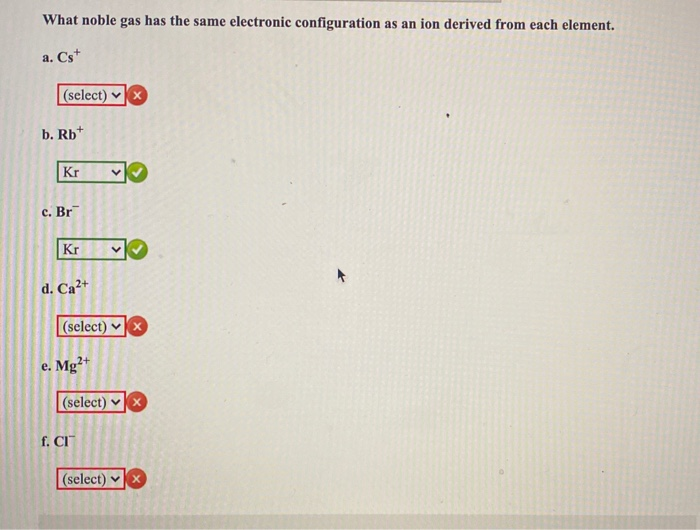
Solved What Noble Gas Has The Same Electronic Configuration Chegg Com

Solved Name The Noble Gas Atom That Has The Same Electron Configuration As Each Of The Ions In The Following Compounds A Barium Sulfide Bas B Strontium Fluoride Srf C Magnesium Oxide Mgo

What Is The Ground State Electron Configuration Of The Al 3 Ion A 1s 2 2s 2 2p 6 3s 2 3p 4 Homeworklib

Webelements Periodic Table Barium Properties Of Free Atoms
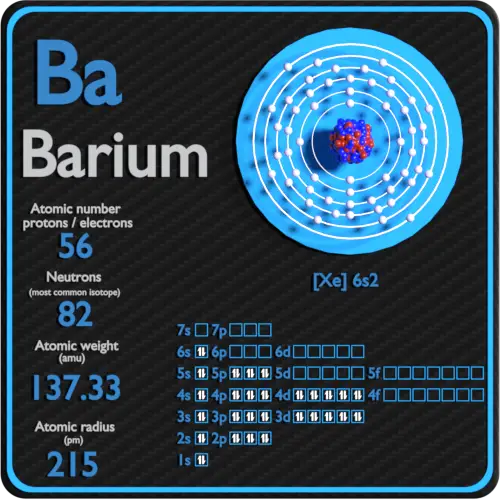
Barium Protons Neutrons Electrons Electron Configuration

Ba 2 Electron Configuration Barium Ion Youtube
What Element Has A Noble Gas Notation Xe 6s2 Socratic

See The Electron Configuration Diagrams For Atoms Of The Elements Electron Configuration Atom Diagram Electrons

Comments
Post a Comment